Components of exhaust gas
Complete combustion of fuels produces only carbon dioxide (CO2) and water, while constituents called traditional “emissions” represent only a small share of the total exhaust gas, below 0.5%(V/V). The major constituents of diesel exhaust are nitrogen, oxygen, water and CO2. Exhaust gas from gasoline fuelled cars does not contain oxygen, because it is consumed in stoichiometric combustion.
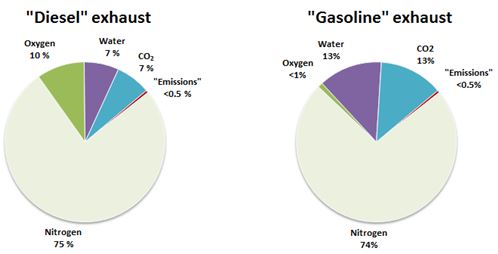
Figure 1. Examples of compositions of the exhaust gases from “diesel” combustion using excess air and stoichiometric spark-ignited “gasoline” combustion. Concentrations are shown as %(V/V).
Air to fuel ratio (AFR) is one of the parameters affecting the exhaust emissions from internal combustion engines (Figure 2). Diesel engines use excess air (lean, AFR>1), while spark-ignited otto engines typically operate close to the stoichiometric air to fuel ratio (AFR~1). In diesel combustion, NOx and PM emissions tend to be elevated, while emissions of THC and CO are typically at low level. Spark-ignited gasoline cars operating close to stoichiometric air to fuel ratio can use TWC, which efficiently reduces CO, HC and NOx emissions. Complicated emission control systems consisting of SCR and diesel particulate filter (DPF) are required for diesel engines to achieve similar emission level as for stoichiometric spark-ignited otto engines. Alternative fuels can be used in both diesel and otto engines. Some alternative fuels can assist performance of existing emission control systems (paraffinic diesel), while others may require development/adjustment of aftertreatment technologies (ethanol, methane).
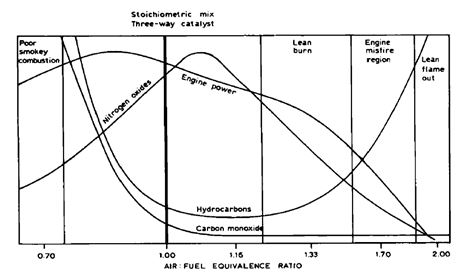
Figure 2. Engine-out emissions from internal combustion engines at different air to fuel ratios (Windawi 1992).
“Emissions”, representing less than 0.5 %(V/V) of the exhaust, include for example the following constituents:
- Carbon monoxide
- Nitrogen oxides and other nitrogen containing compounds, such as NH3 and N2O
- PM consisting of elemental carbon, organic compounds, anions (sulphates, nitrates) and metals
- Hydrocarbons, hundreds of compounds, some of them toxic, for example benzene and 1,3-butadiene, or greenhouse gases, such as methane
- Carbonyls, for example formaldehyde, acetaldehyde and acrolein
- Polycyclic aromatic compounds (PAC), for example PAHs, nitro-PAHs and oxy-PAHs
Most of these emission species are fuel- and engine-dependent, but some of them are formed in emission control devices (NH3, N2O). Methane is emitted from engines and vehicles, particularly when fuelled with methane containing fuels, natural gas or biomethane. N2O is induced by catalyst chemistry of TWC of the spark-ignited gasoline cars. Another catalyst induced emission is NH3, which is associated with harmful effects on health and vegetation, and can form ammonium aerosols that affect climate, and visibility. NH3 originates mainly from agricultural sources, but also from catalytic reactions in TWCs and the urea-based SCR systems for NOx control for diesel vehicles. (Meija-Centeno 2007, EEA 2012b).
IARC classification of diesel engine exhaust is carcinogenic to humans (Group 1), and classification of gasoline exhaust is possibly carcinogenic to humans (Group 2B) (IARC 2013). These classifications are assumedly based on studies with old engines. HEI (2015) found emissions from a 2007 new-technology engine to be not carcinogenic in the rat. A few effects in rat lungs were observed resembling changes seen in earlier studies after long-term exposures to gaseous oxidant pollutants, in particular NO2. The results showed that the 2007 compliant diesel engines equipped with EGR, DOC and DPF significantly reduced levels of PM (90%), VOCs and SVOCs (> 90%) compared with emissions from old engines. The NOx emissions from the 2007 engines were reduced, however, the full extent of NOx reduction was seen for 2010 engines equipped with urea-SCR (>90% reduction compared with 2007 engines).
CO is a colorless, odorless gas, which reduces oxygen delivery to the body's tissues by binding and interfering with heme proteins. The health threat from lower levels of CO is most serious for e.g. persons with impaired cardiovascular systems. Potential effects include damage to the central nervous system, reproduction and prenatal development, and the respiratory system. At sufficient levels, CO is poisonous and can be fatal. (Vallero 2014)
CO contributes to the formation of ground-level ozone, which is harmful to human health and environment.
The sum of nitric oxide (NO) and nitrogen dioxide (NO2), both calculated as NO2, is called nitrogen oxides (NOx). NO is reactive compound, which oxidizes to NO2 in atmosphere, rate of oxidation depends on the conditions and on the other compounds present.
Health - NO2 can irritate the lungs and is addressed with adverse respiratory effects including airway inflammation and increased number and severity of asthma episodes. Inhalation of elevated short-term NO2 concentrations has been associated with increased hospital emergency room visits for respiratory distress (Vallero, 2014). NOx reacts in the atmosphere with various compounds to form toxic products, for example organic nitrates, nitroarenes, nitrosamines and peroxyacetyl nitrate (PAN, a phytotoxicant and mutagen).
Ozone - NOx contribute to formation of ground-level ozone when NOx and VOCs react in the presence of heat and sunlight (see Chapter 3.13).
Acidification, eutrophication - NOx can have adverse effects on terrestrial and aquatic ecosystems through acid rain and eutrophication of waters due to increase in nitrogen containing nutrients. NO2 is a strong oxidizing agent that can form nitric acid (HNO3), which falls to earth as acid rain. Acid rain also deteriorates materials, such as historical buildings and monuments.
PM - NOx and its oxidation products can form small particles in reactions with ammonia, moisture, and other compounds (see PM).
Visibility Impairment - Nitrate particles and NO2 can reduce visibility.
(Websites as in August 2016)
N2O is a nonflammable, colorless gas commonly called “laughing gas”. N2O is used for example as an anesthetic agent. Adverse effects are associated with N2O abuse, such as breathing difficulty.
N2O is a strong greenhouse gas with a global warming potential (GWP, 100-year) 265 relative to CO2. Lifetime of N2O is 121 years. (Myhre et al. 2013 in IPCC AR5).
The N2O probably plays the dominant role in the depletion of stratospheric ozone layer when concentrations of halocarbons reach the pre-industrial concentrations. (Portmann 2012).
NH3 is corrosive and can cause permanent injury in the eye. Dermal exposure to NH3 or its solutions may result in irritation and alkali burns at sufficient concentrations. Ingestion of NH3 solution causes rapid signs and symptoms and extensive alkali burns to the aerodigestive tract. In severe cases perforation of the stomach or oesophagus may occur. Aspiration of ammonia following ingestion may also lead to respiratory complications. Chronic oral exposure to NH3 may lead to osteoporosis secondary to chronic metabolic acidosis. Exposure to a massive concentration of NH3 gas may be fatal within minutes. (Public Health England 2015).
SO2 is a precursor for compounds that are harmful to people and the environment. SO2 is a respiratory irritant, damages crops, and causes visibility problems. SO2 and other sulphur oxides form acids in the atmosphere, particularly sulfuric acid (H2SO4), a key component of acid rain. SO2 also causes crop and material damage when acidic liquid and solid aerosols are deposited. (Vallero 2014).
Volatile organic compounds (VOCs)
In Europe, VOCs are defined as organic compounds having an initial boiling point less than or equal to 250 °C at 101.3 kPa (Directive 2004/42/CE). Examples of VOCs are hydrocarbons, alcohols, ethers, esters and aldehydes. Some of the VOCs are toxic and some contribute to the ozone formation (see ozone). Adverse health effects are identified for example for the following mobile-source VOCs:
- Benzene increases the risks of acute myeloid leukaemia. Benzene seems to affect haematologic indices at exposure concentrations lower than those reported previously. (HEI 2007). IARC (2012) has defined benzene as carcinogenic to humans, Group 1.
- 1,3‑Butadiene has a short lifetime, but it is reactive forming other MSATs, such as formaldehyde, acetaldehyde and acrolein. Concentrations may be elevated in the tobacco smoke. 1,3‑Butadiene may cause lymphohaematopoietic cancers in high-exposures.(HEI 2007). IARC (2012) has defined 1,3-butadiene as carcinogenic to humans, Group 1.
- Formaldehyde is present predominantly indoors, but transport is a source of ambient concentrations, both directly and through photochemical activity. In Brazil, ambient formaldehyde concentrations increased along with increased use of natural gas vehicles. Formaldehyde is an irritant to the eyes, skin and respiratory tract in humans with possible increase in asthma. (HEI 2007). IARC (2012) has defined formaldehyde as carcinogenic to humans, Group 1.
- Acetaldehyde is present in for example in some foods, but it originates also from mobile sources. The use of ethanol as fuel may increase acetaldehyde emissions in air. Acetaldehyde causes irritation to the eyes, skin and respiratory tract and induces cellular inflammation. Acetaldehyde is a carcinogen in rodents, but the data on its carcinogenicity in humans are inadequate. (HEI 2007) IARC has defined acetaldehyde as Group 1 carcinogen when associated with consumption of alcoholic beverages (IARC 2012) Indirect effect of acetaldehyde is through reaction with NOx in the atmospheric photochemical system producing peroxyacetyl nitrate (PAN), which is a phytotoxicant and mutagen.(ref)
- Acrolein is formed from 1,3‑butadiene in atmosphere in addition to the direct sources, such as tobacco smoke. Environmental concentrations have been close to those causing irritation. Acrolein is irritant to the respiratory tract. Chronic inhalation results in inflammation. (HEI 2007). IARC (1995) defined acrolein as “not classifiable as to its carcinogenicity to humans (Group 3 carcinogen).
Particulate mass (PM) and number (PN), and black carbon (BC)
PM from internal combustion engines consists of elemental carbon, organic compounds, metals, sulphates, nitrates and other constituents originating from fuel and lube oil in the combustion process and in the aftertreatment devices. Primary PM forms secondary organic aerosols (SOA) in the atmospheric reactions. Outdoor PM consists also of other species, such as dust and dirt.
Human health concerns of PM include effects on breathing and the respiratory system, damage to lung tissue, and premature death. BC associated in particles increase the global warming potential, while other constituents of PM mainly have a cooling effect on climate.
|
|
|
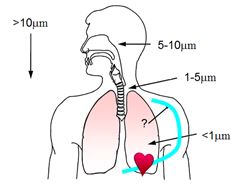
Figure 3. Deposition of particles into human body (Altshuler 2002). |
Effects of PM on human health depend on their size and composition. The primary mechanisms for deposition of particles in the respiratory tract are sedimentation, impaction, and diffusion, which depend on the diameter of the particle. Coarse particles are removed e.g. by swallowing or coughing (Figure 3). Particles below 0.1 μm can reach the surface of the lung. These particles can be removed by scavenging cells (macrophages), but they may also drift into lymphatic vessels, and possibly further into the blood. (DEFRA 2001). Small particles penetrate deeply into the lungs and can cause or worsen respiratory disease such as emphysema and bronchitis, and aggravate existing heart disease.
More than 90% of diesel particles are in the size class below 0.1 μm, ultrafine particles (UPs), and nanoparticles below 50 nm may occur (Figure 4). Diesel particles have a large surface area, which may adsorb various compounds that can be toxic, mutagenic and carcinogenic (e.g., PAHs). (HEI 2002, Kittelson 2002, IARC vol 109, 2016).
Li et al. (2016) found that UPs have detrimental effects on both the cardiovascular and respiratory systems, including a higher incidence of atherosclerosis and exacerbation rate of asthma. UPs can also play a role in allergen sensitization. The inflammatory properties of UPs can be mediated by reactive oxygen species, leading to the generation of proinflammatory cytokines and airway inflammation. In addition, UPs might be able to alter cellular function, penetrate intracellularly and potentially cause DNA damage. (Li et al. 2016).
The toxicological and epidemiological data suggest that the chemical composition of particles may be important contributor to the health effects (Jalava et al. 2009, 2010a).
IARC (Vol 109, 2016) has defined particulate matter of outdoor air pollution as carcinogenic to humans, Group 1.
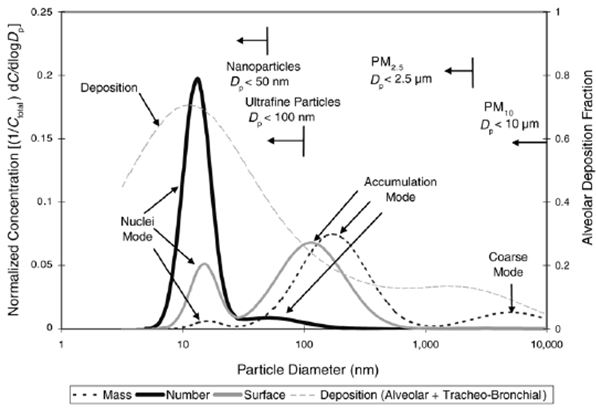
Figure 4. Major features of particle-size distributions from diesel exhaust (IARC vol 109, 2016).
Polyaromatic hydrocarbons (PAHs) and their derivatives
Polycyclic organic matter, POM, defined by the US EPA as “Priority Air Toxic”, consists of hundreds of different compounds, for example PAH and PAHs containing heteroatoms (N, S, O). PAHs are neutral, nonpolar aromatic molecules found in fossil fuels and formed also in incomplete combustion of organic matter (e.g. in engines). Mobile sources may be significant contributors to the ambient PAH concentrations in urban areas. Diesel vehicles are known PAHs emitters, however, gasoline vehicles may also emit high PAH concentrations at cold temperatures (Aakko-Saksa et al. 2011). Cigarette-smoking and food-derived sources, may lead to exposure to PAHs. (HEI 2007).
Naphthalene is the most abundant PAH in ambient air. There is evidence in rodents that exposure to naphthalene leads to inflammation of the nasal tract and tumors of the nasal epithelium. However, there are no data on carcinogenicity in humans. Case reports suggest that exposure may cause effects in blood cells, such as haemolysis and haemolytic anaemia. (HEI 2007).
A few PAHs are potent carcinogens, for example, benzo(a)pyrene is a PAH compound whose metabolites are mutagenic and highly carcinogenic. In general PAHs have been identified as carcinogenic and mutagenic and are considered therefore pollutants of concern with potential health impacts.
The US EPA (1998) defined 16 priority PAHs, many of which are classified as carcinogenic, Group 1, probably carcinogenic, Group 2A or possibly carcinogenic, Group 2B, according to the IARC classification (IARC 2008, 2011). In a list of mobile-source air toxics defined by the US EPA (2007), 7 priority PAHs are included. European directive 2004/107/EC relating to arsenic, cadmium, mercury, nickel and polycyclic aromatic hydrocarbons in ambient air, defines seven priority PAHs: benzo(a)pyrene, benzo(a)anthracene, benzo(b)fluoranthene, benzo(j)fluoranthene, benzo(k)fluoranthene, indeno(1,2,3-cd)pyrene, and dibenz(a,h)anthracene.” These PAHs are classified in Group 2A and Group 2B by IARC. In addition to these definitions, there are several other lists of “Priority PAHs” to describe the cancer-related risks of substances. For example, (Kokko et al. 2000) presented a sum of 14 PAHs based on the US EPA priority list of 16 PAHs, from which naphthalene, acenaphthene and acenaphthylene were excluded, because these low-molecular weight PAHs significantly decrease the repeatability of the results. On the other hand, benzo(e)pyrene was included as it is listed by NIOSH and VDI 3872, for example. Substitued nitro-PAHs and oxy-PAHs may be present in the exhaust gases. For example, the IARC classification for 2-nitropyrene is Group 2A and for 1,8-dinitropyrene Group 2B.
A Working Group of the European Commission prepared a position paper to review knowledge of PAHs in ambient air (EU 2001). As part of the study, toxic equivalency factors (TEFs) relative to benzo(a)pyrene were reviewed. Table 4 summarizes the selected lists for priority PAHs and the TEFs relative to benzo(a)pyrene. Supplementary TEFs are shown for fluorene (Flu) and 7,12-dimethylbenz(a)anthracene (DMBA) (Collins et al. 1998). A total cancer potency for PAHs can be evaluated by calculating the BaP equivalent using Equation (1).
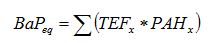 (1)
(1)
BaPeq = Benzo(a)pyrene equivalent (µg/km)
TEFx = Relative toxic equivalency factor for individual PAH compounds in Table 4.
PAHx = Mass emissions (µg/km) of PAH compound
Table 4 summarizes the selected lists for priority PAHs and the toxic equivalency factors relative to benzo(a)pyrene.
Table 1. a) 16 PAHs defined by the US EPA (1998) b) 7 PAHs defined by the US EPA (2007) and c) 7 PAHs defined in European Directive 2004/107/EC. TEF represents toxic equivalency factors relative to benzo(a)pyrene.
|
N |
Ace |
Acy |
Flu |
Phe |
An |
F |
P |
BaA |
DMBA |
Chr |
BbF |
BjF |
BkF |
BaP |
BeP |
IP |
DBahA |
BghiP |
|
|
IARC* |
2B |
3 |
3 |
3 |
3 |
3 |
3 |
2B |
2B |
2B |
2B |
2B |
1 |
3 |
2B |
2A |
3 |
||
|
Ring ** |
2/2 |
3/2 |
3/2 |
3/2 |
3/3 |
3/3 |
4/3 |
4/4 |
4/4 |
4/4 |
4/4 |
5/4 |
5/4 |
5/4 |
5/5 |
5/5 |
6/5 |
5/5 |
6/5 |
|
TEF |
*** |
0.0005– |
0– |
0– |
0– |
0.005– |
0.001– 0.89 |
0.06– |
0.045– |
0.03– |
1 |
0– |
0– |
0.69– |
0.01– |
||||
|
a (16) |
x |
x |
x |
x |
x |
x |
x |
x |
X |
X |
X |
X |
X |
X |
X |
x |
|||
|
c (US 7) |
X |
X |
X |
X |
X |
X |
X |
||||||||||||
|
d (EU7) |
X |
X |
X |
X |
* No. of rings/aromatic rings ** Group 1: carcinogenic; Group 2A: probably carcinogenic; Group 2B: possibly carcinogenic; Group 3: not classifiable with regard to carcinogenicity; Group 4: probably non-carcinogenic.
N= Naphthalene, Ace = Acenaphthene, Acy = Acenaphthylene, Flu = Fluorene, Phe = Phenanthrene, An = Anthracene, F = Fluoranthene, P = Pyrene, BaA = Benz(a)anthracene, DMBA = 7,12-dimethylbenz(a)anthracene, Chr = Chrysene, BbF = Benzo(b)fluoranthene, BkF = Benzo(k)fluoranthene, BjF = Benzo(j)fluoranthene, BaP = Benzo(a)pyrene, IP = Indeno(1,2,3-cd)pyrene, ZBahA = Dibenz(ah)anthracene, BghiP = Benzo(ghi)perylene.