Liquefied petroleum gas (LPG)
Liquefied petroleum gas (LPG) – also known as autogas – is a widely used alternative fuel. LPG is a mixture of propane and butane, and it is being produced as by-product of natural gas and oil refining industry. LPG is a mature, but quite niche alternative fuel that can be used in special spark ignition engines or as an auxiliary fuel in dual fuel compression ignition engines together with diesel oil. LPG is a mixture of propane and butane and it is a by-product of gas and oil industries. The use of LPG in transportation is concentrated in few countries (Korea, Turkey, Russia, and Poland) and it is mainly used in bi-fuel light duty vehicles.
In 2010, LPG was used to power more than 17 million vehicles around the world. Little more than 9% of the global consumption of LPG is used in transportation. (WLPGA). The rest of the LPG is used, for example, in space and water heating, cooking, electricity production and in many industrial processes. The use of autogas is concentrated in a small number of markets: Korea, Turkey, Russia, Poland and Italy accounted for half of world consumption in 2010 and the top ten countries for 75 %. In Korea and Japan, most of the LPG is used in taxis and other light-duty fleet vehicles due to incentives and regulations. In Europe LPG is mostly used in the private sector, in cars which typically have been retrofitted with LPG equipment as opposed to Korea where LPG vehicles are original equipment manufactured (OEM). LPG is not used much in heavy-duty vehicles. (WLPGA). The increasing trend in autogas use can be seen from Figure 1.
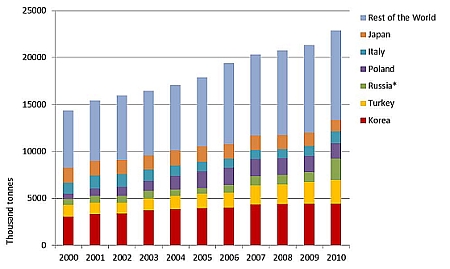
Figure 1. LPG gas use in transportation (WLPGA).


