Fuel properties
Most of current methanol standards related to the automotive sector are available in the US, Italy, Israel, China and India. In Europe, max. 3 vol-% methanol are allowed to be blended in gasoline under the Fuel Quality Directive (2009/30/EC) and CEN standard (EN 228). In the U.S., ASTM D 4814-10a limits methanol up to 0.3 vol-% or up to 2.75 vol-% with an equal volume of butanol, or higher molecular weight alcohol. U.S. EPA waivers under the substantially similar rule allow for methanol levels higher than ASTM D4814-10a, with the “Octamix” waiver allowing a maximum of 5% by volume methanol, with a minimum 2.5 vol-% co-solvents (one or a mixture of higher alcohols including ethanol, propanols, butanols and pentanols). ASTM D5797-07 standards specifying fuel methanol blends (M70- M85) are now being updated by an ASTM Task Force. Specifications for neat methanol exist as well, for example ASTM D-1152/97 and specification of the International Methanol Producers & Consumers Association. In China, a national standard for M85 fuel containing up to 85% of methanol is approved to be used as motor fuel (Green Car Advisor 2009, Methanol Institute 2011). In addition, standards are in place in several provinces in China that govern the use of methanol in various blends with gasoline ranging from 5% to 100%, while a national standard for M15 fuel is in the final drafting stage. Auto manufacturer's recommendations for fuel gasoline qualities in the WWFC 2019 edition states that "Methanol is not permitted". Methanol is included in the rules for marine fuels with low flashpoint (Moirangthem 2016).
Methanol is a clear, colorless, flammable and volatile liquid with an alcoholic odor. It mixes with many organic solvents and in any ratio with water. Some methanol properties are more favourable for use as fuel in internal combustion engines than the others (Schröder et al. 2020):
- High octane number and knocking resistance of methanol is positive feature for otto engines. Methanol has an octane rating significantly higher than that for gasoline.
- Low cetane number makes methanol unsuitable for conventional diesel engines without modifications of fuel or engine.
- High volatility of methanol in blends but low vapor pressure at high concentrations affect cold-start performance of engines and evaporative emissions (see discussion below Table 1).
- The high heat of vaporization of methanol cools down the intake air, which allows the combustion of higher amount of fuel. Hence, the compression ratio of engines can be increased and smaller, more economic, high performance engines can be designed.
- No carbon-to-carbon bonds and high oxygen content of methanol leads theoretically to soot-free combustion. Additionally, internal engine measures such as exhaust gas recirculation (EGR) can reduce NOx emissions efficiently in compression ignition engine concepts. On the other hand, incomplete combustion of methanol may produce more formaldehyde and acetic acid emissions, which could be controlled by oxidation catalyst.
- Low lean flammability limit
- Low volumetric energy content of methanol compared with that of gasoline, diesel or ethanol reduce the driving range of the vehicle, if not compensated with larger tanks or high efficiency engines. The engine fuel system needs to be modified due to the lower heating value.
- Phase separtion issues of methanol blends with gasoline are discussed below Table 1.
- Poor miscibility with diesel
- Tendency to evaporate in fuel lines
- Corrosive and chemical degradation of materials
- Poor lubrication properties of methanol and degradation of oil lubrication properties may lead to an increased wear on engine fuel systemcomponents. Lubricity additives are needed.
Table 1 shows selected fuel properties of methanol.
Table 1. Selected fuel properties of methanol.
|
|
Methanol |
|
Formula |
CH3OH |
|
Molecular weight, g/mol |
32.0 |
|
Carbon/Hydrogen/Oxygen, wt-% |
37.5/12.5 /49.9 |
|
Methanol purity, wt-% |
>99.7 |
|
Water, wt-% |
<0.1 |
|
Chlorides as CL ion, ppm |
<0.5 |
|
Sulfur, ppm |
<0.5 |
|
Density at 15 °C, kg/dm3 |
0.796 |
|
Dynamic viscosity at 20 °C, cP |
0.544 a |
|
Kinematic viscosity at 20 °C, m2 /s |
7.37 x 10-7 |
|
Boiling point, °C |
64.6 |
|
Freezing point, °C |
-97.6 |
|
Flash point (closed vessel), °C |
11, 12 |
|
RON, neat |
107 - 109 |
|
Blending RON * |
127 - 136 |
|
Blending MON * |
99 - 104 |
|
Cetane number ** |
3 |
|
Neat vapor pressure at 37.8°C, kPa |
32 |
|
Blending vapor pressure at 37.8 °C, kPa * |
214 |
|
LHV heating value, MJ/kg (MJ/l) |
20.0 (15.9) |
|
Heat of vaporization, kJ/kg |
1160 - 1174 |
|
Heat of combustion, net, kJ/kg |
19 930 |
|
Heat capacity (25 °C, 101.3 kPa), Jmol-1 K-1 - liquid / vapor |
81.08 / 44.06 |
|
Heat of combustion, net, kJ/kg |
19 930 |
|
Self-ignition temperature, °C |
464, 470 |
|
Ignition limits, fuel in air, vol-% |
7 - 36 (6 - 36.5) |
|
Stoichiometric air to fuel ratio |
6.4 |
|
Solubility in water |
fully miscible |
|
Octanol partition coefficient, kow |
-0.82 |
|
Odor threshold in air, mean ppm |
160 |
|
Critical temperature (°C), pressure (MPa) and density (g/cm3) |
239 / 8.084 / 0.2715 |
|
Critical compressibility factor |
0.224 |
|
Surface tension at 25 °C, mNm -1 |
22.07 |
|
Refractive index at 25 °C |
1.32652 |
|
Self-ignition temperature, °C |
464 - 470 |
|
Thermal conductivity, mWm-1 K-1 - liquid at 25 °C / vapor at 100 °C |
200 / 14.07 |
|
Coefficient of cubic thermal expansion, per °C |
0.00149 (20 °C) 0.00159 (40 °C) |
|
Flame spread rate, m/s |
2 - 4 |
|
Laminar flame speed (1 bar, 300 K), m/s |
0.50 |
*Methanol Institute, Graboski 2003, Owen 1995, Bromberg and Cheng (2010), Bechtold 1997
a Kinematic viscosity = dynamic viscosity/density; kV = (0.544 x 10-3 kg/ms)/(796 kg/m3 ) = 7 x 10-7 m2 /s
The blending vapor pressure of methanol is high. This means that despite the low vapor pressure of neat methanol (32 kPa at 37.8 °C), the addition of methanol into gasoline results in an increase in vapor pressure of the blended fuel (Figure 1). This is due to the capability of methanol to form azeotropes with hydrocarbons of gasoline. However, when blending ratio of alcohol increases, vapor pressure gradually declines as illustrated in Ethanol chapter.
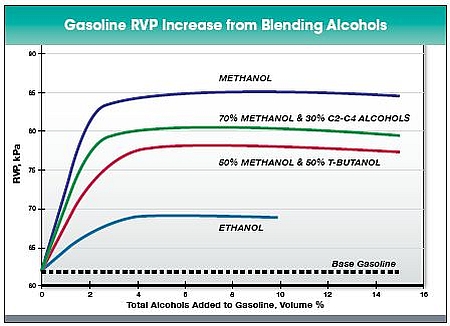
Figure 1. Increase of vapor pressure when blending methanol, ethanol, and alcohol blends with gasoline. (Methanol Institute a).
Methanol is more prone to phase separation than ethanol when blended with gasoline (Ethanol chapter). Methanol requires co-solvents to be blended with gasoline even at low concentrations to provide a stable fuel blend. Examples of possible co-solvents are isopropanol and tertiary butanol (Figure 2).
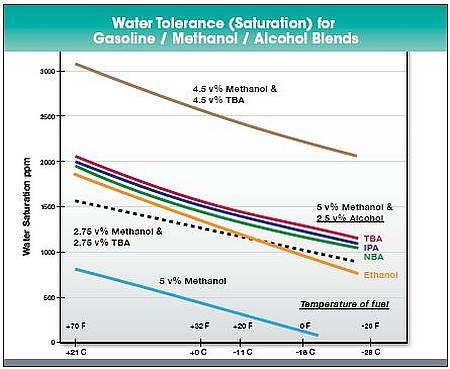
Figure 2. Water tolerance of methanol with co-solvents in gasoline. TBA = tertiary butanol; NBA = normal butanol; IPA = isopropanol. (Methanol Institute a).


