Methanol
Raw materials - Methanol is typically produced from natural gas or coal, but it can also be renewable methanol made from biomass. Methanol is sometimes called “wood alcohol”, because it was once produced as a byproduct of wood distillation. (Methanol Institute). Biomethanol is also produced from glycerine, which is a byproduct from production of Fatty Acid Methyl Esters (FAME) (BioMCN). In Iceland, renewable electro-fuel synthetic methanol is produced from geothermal CO2 and renewable hydrogen by Carbon Recycling International (CRI). Some small-scale production facilities for renewable methanol are operating or planned.
Climate impact - Even when produced from natural gas, methanol has a slight greenhouse gas (GHG) emission benefit over gasoline. For renewable methanol, GHG emissions are potentially relatively low In comparison with the default values of the European Renewable Energy Directive, RED II (2021 onwards). Overall, GHG reduction potentials of renewable methanol produced on an industrial scale can be competitive to established renewable fuels, if using suitable resources like waste wood and cultivated wood. (Schröder et al. 2020).
Volume - Methanol is one of the most common chemicals globally. Driven by Chinese demand growth, global methanol demand have increased from 61 to 125 million metric tons from 2012 to 2016 Production capacity of sustainable renewable methanol is only less than 1 million tonnes per year. (IHS global market study, Schröder et al. 2020).
Price - Methanol prices are competitive with gasoline prices, even when considered on an energy equivalent basis (Bromberg and Cheng 2010). Renewable methanol is usually more expensive than fossil methanol similarly to other renewable fuels. When considering production of advanced renewable fuels, methanol is one of the most cost-efficient options. One possibility to reduce costs of methanol is to use a lower purity than 99.85% required for the chemical industry. Combustion engines operate even when purity of methanol is 90% and with high water content. (Schröder et al. 2020).
Legislation, standards and properties
Standards – Most of current methanol standards related to the automotive sector are available in the US, Italy, Israel, China and India. In Europe, max. 3 vol-% methanol are allowed to be blended in gasoline under the Fuel Quality Directive (2009/30/EC) and CEN standard (EN 228). In the U.S., ASTM D 4814-10a limits methanol up to 0.3 vol-% or up to 2.75 vol-% with an equal volume of butanol, or higher molecular weight alcohol. U.S. EPA waivers under the substantially similar rule allow for methanol levels higher than ASTM D4814-10a, with the “Octamix” waiver allowing a maximum of 5% by volume methanol, with a minimum 2.5 vol-% co-solvents (one or a mixture of higher alcohols including ethanol, propanols, butanols and pentanols). ASTM D5797-07 standards specifying fuel methanol blends (M70- M85) are now being updated by an ASTM Task Force. Specifications for neat methanol exist as well, for example ASTM D-1152/97 and specification of the International Methanol Producers & Consumers Association. In China, a national standard for M85 fuel containing up to 85% of methanol is approved to be used as motor fuel (Green Car Advisor 2009, Methanol Institute 2011). In addition, standards are in place in several provinces in China that govern the use of methanol in various blends with gasoline ranging from 5% to 100%, while a national standard for M15 fuel is in the final drafting stage. Auto manufacturer's recommendations for fuel gasoline qualities in the WWFC 2019 edition states that "Methanol is not permitted". Methanol is included in the rules for marine fuels with low flashpoint (Moirangthem 2016).
Fuel properties – Methanol is a clear, colorless, flammable and volatile liquid with an alcoholic odor. It mixes with many organic solvents and in any ratio with water. Some methanol properties are more favourable for use as fuel in internal combustion engines than the others (Schröder et al. 2020):
- High octane number and knocking resistance of methanol is positive feature for otto engines. Methanol has an octane rating significantly higher than that for gasoline.
- Low cetane number makes methanol unsuitable for conventional diesel engines without modifications of fuel or engine.
- High volatility of methanol in blends but low vapor pressure at high concentrations affect cold-start performance of engines and evaporative emissions (see discussion below Table 1).
- The high heat of vaporization of methanol cools down the intake air, which allows the combustion of higher amount of fuel. Hence, the compression ratio of engines can be increased and smaller, more economic, high performance engines can be designed.
- No carbon-to-carbon bonds and high oxygen content of methanol leads theoretically to soot-free combustion. Additionally, internal engine measures such as exhaust gas recirculation (EGR) can reduce NOx emissions efficiently in compression ignition engine concepts. On the other hand, incomplete combustion of methanol may produce more formaldehyde and acetic acid emissions, which could be controlled by oxidation catalyst.
- Low lean flammability limit
- Low volumetric energy content of methanol compared with that of gasoline, diesel or ethanol reduce the driving range of the vehicle, if not compensated with larger tanks or high efficiency engines. The engine fuel system needs to be modified due to the lower heating value.
- Phase separtion issues of methanol blends with gasoline are discussed below Table 1.
- Poor miscibility with diesel
- Tendency to evaporate in fuel lines
- Corrosive and chemical degradation of materials
- Poor lubrication properties of methanol and degradation of oil lubrication properties may lead to an increased wear on engine fuel systemcomponents. Lubricity additives are needed.
Table 1 shows selected fuel properties of methanol.
Table 1. Selected fuel properties of methanol.
|
|
Methanol |
|
Formula |
CH3OH |
|
Molecular weight, g/mol |
32.0 |
|
Carbon/Hydrogen/Oxygen, wt-% |
37.5/12.5 /49.9 |
|
Methanol purity, wt-% |
>99.7 |
|
Water, wt-% |
<0.1 |
|
Chlorides as CL ion, ppm |
<0.5 |
|
Sulfur, ppm |
<0.5 |
|
Density at 15 °C, kg/dm3 |
0.796 |
|
Dynamic viscosity at 20 °C, cP |
0.544 a |
|
Kinematic viscosity at 20 °C, m2 /s |
7.37 x 10-7 |
|
Boiling point, °C |
64.6 |
|
Freezing point, °C |
-97.6 |
|
Flash point (closed vessel), °C |
11, 12 |
|
RON, neat |
107 - 109 |
|
Blending RON * |
127 - 136 |
|
Blending MON * |
99 - 104 |
|
Cetane number ** |
3 |
|
Neat vapor pressure at 37.8°C, kPa |
32 |
|
Blending vapor pressure at 37.8 °C, kPa * |
214 |
|
LHV heating value, MJ/kg (MJ/l) |
20.0 (15.9) |
|
Heat of vaporization, kJ/kg |
1160 - 1174 |
|
Heat of combustion, net, kJ/kg |
19 930 |
|
Heat capacity (25 °C, 101.3 kPa), Jmol-1 K-1 - liquid / vapor |
81.08 / 44.06 |
|
Heat of combustion, net, kJ/kg |
19 930 |
|
Self-ignition temperature, °C |
464, 470 |
|
Ignition limits, fuel in air, vol-% |
7 - 36 (6 - 36.5) |
|
Stoichiometric air to fuel ratio |
6.4 |
|
Solubility in water |
fully miscible |
|
Octanol partition coefficient, kow |
-0.82 |
|
Odor threshold in air, mean ppm |
160 |
|
Critical temperature (°C), pressure (MPa) and density (g/cm3) |
239 / 8.084 / 0.2715 |
|
Critical compressibility factor |
0.224 |
|
Surface tension at 25 °C, mNm -1 |
22.07 |
|
Refractive index at 25 °C |
1.32652 |
|
Self-ignition temperature, °C |
464 - 470 |
|
Thermal conductivity, mWm-1 K-1 - liquid at 25 °C / vapor at 100 °C |
200 / 14.07 |
|
Coefficient of cubic thermal expansion, per °C |
0.00149 (20 °C) 0.00159 (40 °C) |
|
Flame spread rate, m/s |
2 - 4 |
|
Laminar flame speed (1 bar, 300 K), m/s |
0.50 |
Volatility - The blending vapor pressure of methanol is high. This means that despite the low vapor pressure of neat methanol (32 kPa at 37.8 °C), the addition of methanol into gasoline results in an increase in vapor pressure of the blended fuel (Figure 1). This is due to the capability of methanol to form azeotropes with hydrocarbons of gasoline. However, when blending ratio of alcohol increases, vapor pressure gradually declines as illustrated in Ethanol chapter.
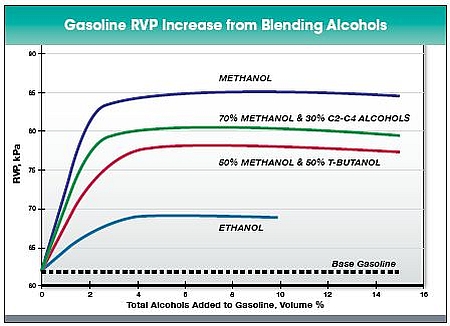
Figure 1. Increase of vapor pressure when blending methanol, ethanol, and alcohol blends with gasoline. (Methanol Institute a).
Phase separation - Methanol is more prone to phase separation than ethanol when blended with gasoline (Ethanol chapter). Methanol requires co-solvents to be blended with gasoline even at low concentrations to provide a stable fuel blend. Examples of possible co-solvents are isopropanol and tertiary butanol (Figure 2).
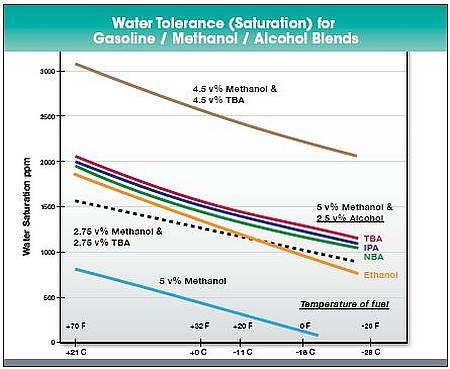
Figure 2. Water tolerance of methanol with co-solvents in gasoline. TBA = tertiary butanol; NBA = normal butanol; IPA = isopropanol. (Methanol Institute a).
Materials - When considering methanol use as gasoline component, corrosion inhibitors, co-solvents, and alcohol compatible materials in vehicles are needed to resist phase separation, maintain stability and safety. In contrast to hydrocarbons, methanol is a polar molecule and thus corrosive to individual metals and alloys as well as elastomers and polymers that are widely used in engine fuel systems and fuel distribution chain designed for conventional hydrocarbon fuels.
Recommended materials for methanol depend on the purpose of use. Elastomers and polymers that are not recommended include fluorosilicone (FVMQ), fluororubber (FPM, FKM), hydrogenated nitrile butadiene rubber (HNBR), neoprene (CR), nitrile butadiene rubber (NBR), polyurethane (PUR) and polyvinyl chloride (PVC). Metals that are not compatible with methanol are aluminium, copper, titanium, zinc and some of their alloys depending on their purpose. Electrical conductivity of methanol increase risks for galvanic corrosion of some metals. (Schröder et al. 2020). According to Bromberg and Cheng (2010) methanol fuels can be aggressive towards magnesium, and if water is present, towards aluminum. Corrosion inhibitor additives and formulated engine oils reduce corrosive effects of methanol.
Safety - Neat methanol burns with an invisible flame, which is a safety risk aspect. Otherwise methanol may be considered even as a safer fuel than gasoline, harder to ignite, slower burning, and producing one-eighth the heat of gasoline.
Methanol, like all transportation fuels is toxic and should not be ingested. Methanol is readily biodegradable in both aerobic and anaerobic environments, and with a half-life in ground and surface water of one to six days. Further information for safe storage of methanol are documented in a Technical bulletin for methanol drums and the Methanol Safe Handling Manual of the Methanol Institute (MI 2016 and 2017).
Methanol was the primary alternative fuel considered for transport sector in 1970’s and 1980’s to reduce dependence on fossil oil. Methanol was used as a transportation fuel until the mid-1990s in North America and Europe. In China’s transportation fuel pool, various methanol blends ranging from M5 to M100 are used. In China, mass production of methanol vehicles is capable of producing up to 500,000 engines for M100. Methanol-blended fuels are explored also by many other countries, for example Denmark (Task 56 report).
Methanol can be used as low or high concentration blends for road transport and marine applications, with conventional or special engine technologies (Table 2). AMF Task 56 report presents in detail methanol use options for different transport sectors. Here a condensed view is given,
Table 2. Methanol engine concepts (Schröder et al. 2020).
|
Engine type |
Mode |
Fuel |
Transport sector |
|
Spark ignition (SI) |
Port fuel injection (PFI) Direct injection (DI) Direct injection (DI lean) |
M0 to M85, GEM, MTBE* |
PC, LDV, HDV |
|
Compression ignition (CI) |
Dual fuel (DF) |
M0-M50 |
HDV, Marine |
|
Direct injection (DI) |
M100, MD95, FAME* |
HDV, Marine |
|
|
New concepts (HCCI, PPC) |
M100 |
HDV, Marine |
|
|
Fuel cell (FC) |
|
M100 |
PC, LDV, HDV, Marine |
*Fuel components produced from methanol
Spark ignition engines – Fuel properties of methanol resemble more gasoline than diesel (see fuel properties section). However, methanol blending in gasoline is limited to low concentrations for use in conventional SI engines, for example in Europe and North America, up to a few percentages. Infrastructure and cars are not designed for methanol use in these regions. Methanol is compatible with conventional gasoline in a form of ether, MTBE. Due to high octane rating of methanol, it is used for race cars and for some other specialty engine applications.
High methanol concentrations, e.g. M85, is used in special Flexible Fuel Vehicles (FFVs), which were first developed for methanol and later optimized for ethanol. Mid-level (A20-A30) and high-level alcohol fuels are high octane fuels that would allow automakers to optimize cars with higher engine compression ratios, downsized engines, increased turbocharging, and enhanced direct injection. High engine efficiency could compensate for methanol’s low energy density. Bromberg and Cheng (2010) have highlighted the potential of directly injected spark ignited engines for heavy-duty vehicles.
In AMF Task 44 (Fanand Donglian 2017), methanol blends (M15 and M30) were studied in comparison with neat gasoline using two PFI and two GDI vehicles. The tests were conducted at normal (25℃) and at low ambient temperatures (-7℃). Also ethanol blends were studied, but those results are not referred here. Many emission components were high during the first acceleration, but reduced to nearly zero as the catalyst lighted off. In both test temperatures, hydrocarbons (HC), carbon monoxide (CO) and methane emissions decreased slightly as the alcohol proportion of fuel increased, while nitrogen oxides (NOX) increased slightly. Tailpipe CO2 did not change substantially. Unburned methanol, formaldehyde and acetaldehyde emissions increased proportionally with the increasing alcohol content, while benzene, toluene, ethylene, propylene, 1,3-butadiene and isobutene decreased slightly. In the evaporative emission tests, only slight differences in the HC emissions were observed between M15 and gasoline.
In the earlier work, increasing methanol content of fuel has reduced CO, HC and NOx when compared to gasoline, while formaldehyde emissions have increased, especially at cold-starts (Bromberg and Cheng 2010, Bechtold et al. 2007 and Ohlström et al. 2001).
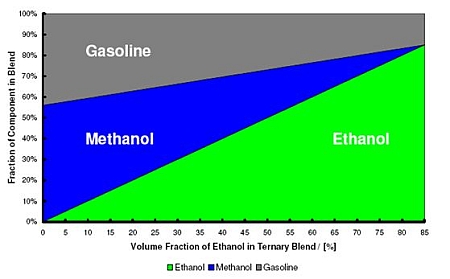
In some markets, the focus is on blends of gasoline, ethanol and methanol (GEM). In this concept, ethanol is serving as a co-solvent for methanol. These tri-component blends have constant air-to-fuel ratio of 9.7:1, which is same ratio as air-to-fuel ratio for E85 fuel (Figure 3). Behaviour of virtual and physical alcohol sensors used in the FFVs have been studied with GEM blends, as well as performance of cars at cold temperatures, emissions and costs. The results indicate that GEM blends could be used in FFV cars as a drop-in alternative to E85 fuel (Turner et al. 2012).
Figure 3. Gasoline, ethanol, methanol blends (GEM) with air-to-fuel ratio equivalent to conventional E85 fuel. (Turner et al. 2012).
Compression ignition engines – Fuel properties of methanol do not resemble those of diesel fuel, for example, cetane number of methanol is extremely low. However, there are options to use methanol in CI engines. One option to use methanol in conventional diesel engines in to convert it to Fatty Acid Methyl Esters (FAME). Other options cover adding an ignition enhancer to methanol, so called MD95 concept and dual-fuel methanol diesel engine concept. Other options are described by Schröder et al. (2020).
MD95 concept – A concept of adding an ignition enhancer to methanol was studied in AMF Task 46 (Nylund et al. 2016) using Scania ethanol engine and additized methanol and later in SUMMETH project for marine sector (Aakko-Saksa 2020). This engine concept proved to operate quite well with additive treated neat methanol. However, with these methanol fuels the engine didn’t reach full power, and the injection periods on partial load were prolonged compared to ethanol operation. Methanol increased the particulate matter (PM) emissions, which was thought to be an indication of semivolatile components or artifacts as methanol contains no soot forming carbon-to-carbon bonds. The standard oxidation catalyst most probably would have reduced this PM with methanol. A blend of 70 % ethanol and 30 % methanol delivered lower CO, HC and NOx emissions compared to the baseline ethanol fuel. However, it must be stated that Scania doesn’t approve the use of methanol fuels.
Dual-fuel engines - In marine sector, methanol is regarded as promising marine fuel alternative (Moirangthem 2016, Ellis and Tanneberger 2015). Also engines for methanol use in marine sector are available. Wärtsilä has developed a methanol-diesel retrofit concept, a dual-fuel technology, which has the advantage of using diesel as a back-up fuel. Common rail system for methanol injection, cylinder heads, fuel injectors and changes in fuel pumps are needed, amongst others. (Haraldson 2013). This concept is demonstrated in Stena Germanica in Sweden. MAN has developed a methanol engine technology that is used in seven 50,000 dwt tankers by Waterfront Shipping in Canada. The alternatives for marine sector were discussed in the AMF Task 41 (McGill et al. 2013).
Development is ongoing on partially premixed combustion (PPC), in which lowered combustion temperature limits NOX formation and enables high efficiency when using 100% methanol without ignition enhancer (Schröder et al. 2020).
Generally, the special engine technologies discussed for ethanol can be considered also for methanol use, as discussed in Task 44, 46, 54 and 56. Further discussion is presented in Task 56 report including methanol use in fuel cell electric vehicles (Schröder et al. 2020).
References
Bechtold, R. L. (1997) Alternative Fuels Guidebook - Properties, Storage, Dispensing, and Vehicle Facility Modifications. Society of Automotive Engineers, Inc.
Bechtold, R., Goodman, M. and Timbario, T. (2007) Use of Methanol as a Transportation Fuel. Report prepared for Methanol Institute.
BioMCN 2013 (http://www.biomcn.eu/our-product/bio-methanol.html)
Bromberg, L. and Cheng, W. (2010) Methanol as an alternative transportation fuel in the US: Options for sustainable and/or energy- secure transportation. Final report. UT-Battelle Subcontract Number:4000096701
Ellis, J. and Tanneberger. K. (2015) Study on the Use of Ethyl and Methyl Alcohol as Alternative Fuels in Shipping Report Prepared for the European Maritime Safety Agency (EMSA ). Final Report.
Fan, Z. and Donglian, T. (Eds.) (2016) Research on Unregulated Pollutants from Alcohol-Fuelled Vehicles. AMF Task 44 report.
Graboski, M. (1999) Tautomerism and octane quality in carbonyl-containing oxygenates. Ind. Eng. Chem. Res. 38(1999)3776-3778.
Green Car Advisor (2009) China Approves Methanol as Clean-Burning Alternative to Gasoline. 10 November 2009. blogs.edmunds.com.
Haraldson, L. 2014. “Methanol as a Marine Fuel - Engine Manufacturers’ Perspective.” In Methanol as a Marine Fuel Seminar, 8 May 2014. Gothenburg.
Landälv, I. (2017) Methanol as a Renewable Energy Resource - a Knowledge Synthesis. The Swedish knowledge centre for renewable transportation fuels. Report f3 2015:08. http://www.f3centre.se/sites/default/files/f3_2015-08_landalv_final_170918_0.pdf.
McGill, R., Remley, W. and Winther. K. (2013) Alternative Fuels for Marine Applications. AMF Task 41 report.
Methanol Institute (2011). online www.methanol.org.
Methanol Institute a. Methanol Institute's Technical Product Bulletin: Methanol Use in Gasoline - Blending, Storage and Handling of Gasoline Containing Methanol. Available online: http://www.methanol.org/.../Blending-Handling-Bulletin-(Final).aspx
Methanol Institute b. Methanol Institute's Technical Product Bulletin: Methanol Gasoline Blends - Alternative Fuel For Today's Automobiles and Cleaner Burning Octane For Today's Oil Refinery. Available online: http://www.methanol.org/.../Blenders-Product- Bulletin-(Final).aspx
Methanol Institute, 2017. Medina E, Wellon GC, Evergren F. Methanol safe handling manual. 4th ed.
Moirangthem, K. (2016) Alternative Fuels for Marine and Inland Waterways. European Commission - Joint Research Centre Technical Reports. doi:10.2790/227559.
Nichols, R. (2003) The Methanol Story: A Sustainable Fuel for the Future. Journal of Scientific & Industrial Research. Vol. 62, p. 97-105.
Nylund, N.-O., Murtonen, T., Westerholm, M., Söderström, C., Huhtisaari, T. and Singh, G. 2015. “Testing of Various Fuel and Additive Options in a Compression-Ignited Heavy-Duty Alcohol Engine.” In The 21st International Symposium on Alcohol Fuels, 10 March 2015 , Gwangju, Korea., 1–15. AMF Task 46 report.
Ohlström, M., Mäkinen, T., Laurikko, J. and Pipatti, R. (2001) New concepts for biofuels in transportation. Biomass-based methanol production and reduced emissions in advanced vehicles. VTT Research Notes 2074.
Owen, K. and Coley, T. (1995) Automotive fuels reference book. 2nd edition. Warrendale, USA: Society of Automotive Engineers. 963 p. ISBN 1-56091-589-7.
Rosenblatt, D. and Karman, D. (2020) GDI Engines and Alcohol Fuels. AMF Task 54 report.
SAE (2007) Alternative Automotive Fuels. Surface Vehicle Information Report J1297. Society of Automotive Engineers.
Schröder, J., Müller-Langer, F., Aakko-Saksa, P. Winther, K. Baumgarten, W. and Lindgren, M. (2020) Methanol as Motor Fuel - Summary Report. AMF Task 56 report.
Turner, J.W.G., Pearson, R.J., McGregor, M.A., Ramsay, J.M., Dekker, E., Iosefa, B., Dolan, G.A., Johansson, K. and Bergström, K. ac (2012) GEM Ternary blends: Testing Iso-stoichiometric mixtures of gasoline, ethanol, and methanol in a production Flex-Fuel Vehicle Fitted with a Physical Alcohol Sensor. Society of Automotive Engineers. SAE Technical Paper 2012-01-1279.