Fuel properties
A number of different oxygenates, for example alcohols, ethers, esters, and carbonates, have been explored as diesel fuel components (Table 1). Many studies have covered a comprehensive set of oxygenates, e.g. Pecci et al. (1991) reviewed some 80 oxygenates, Natarajan et al. (2001) 71 oxygenates and Delfort et al. (2002) 18 oxygenates. In addition, numerous studies have focused on specific oxygenate groups (Nylund et al. 2005).
Table 1. The general structure of oxygenates
| R-OH | Alcohols |
| R-O-R | Ethers |
| R-O-R-O-R | Glycol ethers |
| R-O-C-O-R | Acetals |
| R-C(=O)-O-R | Esters |
| R-O-C(=O)-O-R | Carbonates |
| R hydrocarbon chain; C carbon; O oxygen | |
Many fuel properties of oxygenates need consideration when evaluated as diesel fuel components, for example cetane number, flammability, volatility, density, viscosity and lubricity. Oxygenates are generally polar in nature, which may lead to compatibility problems when blended with diesel fuel. The most common diesel oxygenates are fatty acid esters. Low molecular weight oxygenates are typically not compatible with diesel fuel, whereas some heavier alcohols, ethers, and acetals are considered as promising diesel blending components. Special engines might be capable to use "non-compatible" oxygenates, however, an optimum oxygenate would be compatible with existing engines and infrastructure.There are a number of fuel properties, both physical and chemical, which are important for the proper operation of a diesel engine. Regulations and standards control diesel fuel quality, and these requirements should be met also by when oxygenates are blended in diesel fuel.
Fuel properties of oxygenates depend on, e.g., length and type of alkyl chains. Oxygenates with higher molecular weight often have higher density, higher boiling point, higher viscosity, better lubricity, lower volatility, and lower flammability than respective oxygenates with lower molecular weight. Therefore, oxygenates with higher molecular weight are preferred as diesel fuel components.
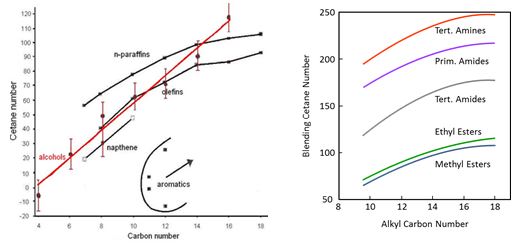
The diesel process is based on auto-ignition of the fuel, which is described by cetane number. In general, poor ignition quality leads to improper combustion and increased exhaust emissions. Ignition improver additives may increase cetane number to some extent. A minimum cetane number of 51 is defined for diesel fuel in Europe. As concerns hydrocarbons, normal alkanes, called straight-chain paraffins, have the highest cetane numbers, whereas aromatics have the lowest cetane numbers. Ignition quality improves as the chain length increases (Figure 1). This applies also to oxygenates with same functional groups and similar branching of alkyl chain. Blending cetane number is reported to increase with increasing alkyl chain up to di-n-hexyl ether. Branching of the alkyl chain depresses cetane number of oxygenates. High cetane numbers have been observed for molecules with three ether groups in structure, and for molecules with oxygen atom at central or at omega positions. Cetane numbers of esters are quite modest. (Pecci et al. 1991, Stournas et al. 1993).
Figure 1. The effect of molecular size on cetane number (Shell Lexikon, Stournas et al. 1993).
Distillation range of diesel fuel is around 180 - 340 °C and new components are desired to fall into this range to ensure proper combustion in diesel engine. However, traditional distillation methods may not be applicable for oxygenates (Smith et al. 2008). Storage and handling regulations for fuels are based on the flash point, which is the limit temperature for the formation of an ignitable air-fuel vapor mixture. In Europe, flash point of diesel fuel is higher than 55 °C.
Hydrocarbons, especially alkanes, are non-polar compounds, whereas oxygenates are polar in nature. The most polar oxygenates, e.g. methanol and ethanol, are not miscible with diesel fuel with exception of emulsions. Less polar oxygenates, for instance n-pentyl ether, are miscible with diesel fuel. Miscibility depends on the chemical structure, oxygen content, and on the physical properties of substances (“like dissolves like”). Increasing aromatics content of diesel fuel improves the solubility of oxygenates with diesel fuel. (McCormic 2001). Water affinity of oxygenates is higher than that of hydrocarbons. In addition, oxygenates tend to be hygroscopic absorbing water from ambient air. Dissolved water is not harmful for an diesel engine, whereas phase separation presents a considerable risk. Also corrosion of materials may occur with oxygenates. For many oxygenates lubricity is a problem. This can be alleviated by using additives. Some oxygenates may form aggressive compounds, such as acids.
Diesel fuel injection system is based on volumetric principle. Therefore lower density fuel typically leads to reduced maximum power output and higher volumetric fuel consumption. For oxygenates, energy content is typically lower than that for hydrocarbon fuels. Low viscosity of fuel may cause fuel leakages, whereas high viscosity of fuel may overload the injection system. Typically, oxygenates do not contain aromatics or sulfur, which is a benefit as regards exhaust emissions and exhaust aftertreatment devices. Cold properties of oxygenates improve with increasing branching, and worsen with increasing number of oxygen atoms. (Pecci et al. 1991)