Compatibility
It is difficult to rate best diesel oxygenates based on miscellaneous, partly contradictory results. However, many promising oxygenates have been found, such as alcohols (C9-C12), ethers (DNPE), maleates (DBM), glycol ethers (TPGME) and carbonates. Particularly low PM emissions have been achieved for oxygenates blended with paraffinic fuel (Frijters et al. 2006). In this Chapter, selected examples of oxygenates considered as diesel components are referred.
Esters and derivatives
One of the esters considered as diesel fuel components is ethyl levulinate, which can be produced from cellulosic materials. However, cetane number of neat ethyl levulinate is poor, below 10. (McCormic 2002, Biofine Inc.).
2-Methoxyethyl acetate (MEA) at 20% blend in diesel fuel reduced smoke, CO and HC emissions, but had only slight effect on NOx emission in the tests with a single-cylinder engine. The ignition delay and duration of combustion were shorter for MEA than for diesel fuel. (Yanfeng et al. 2007). Hilden et al. (2001) reported that di-isobutyl adibate resulted in lower exhaust emissions than di-butyl phthalate. Lin and Huang (2003) reported that NOx, CO, and CO2 emissions decreased, but brake-specific fuel consumption increased with the ethylene glycol mono-acetate as a fuel component for marine diesel engines.
Blending cetane numbers of fatty acid derived amides (R-C(=O)NR´R´´) and amines (R-NH2) are high (Figure 1). For tertiary fatty amides at 5% concentration in diesel fuel, PM emissions reduced in the tests with a Petter engine. (Stournas 1993).
Glycerol is formed as a side-product in FAME production. Glycerol derivatives, such as glycerol-tert-butyl ethers (GTBE) have been studied as diesel fuel components. With di-butoxy glycerol, PM emissions decreased, but NOx emissions increased. (Di Serio et al. 2010, García et al. 2008, Boot et al. 2007, Spooner-Wyman et al. 2003, Karas et al. 1994).
Alcohols
Butanol isomers are lower-boiling compounds (82 - 118 °C) than diesel fuel, and primarily considered as gasoline blending components. With 10 vol-% butanol in diesel fuel, cetane number and lubricity fall below requirements. When fuel contains also 10 vol-% FAME, cetane number, viscosity and lubricity may be sufficient, but flash point remains low (44 °C). 20% and 40% blends of n-butanol showed low smoke and NOx emissions in a common rail, direct-injection EGR equipped diesel engine (Valentino et al. 2012, Wadumesthrige et al. 2010, Zöldy et al. 2010).
A blend containing 5% of rapeseed methyl ester and 10% of heavy alcohols, called Agrodiesel 15, is a stable solution meeting diesel standards. Lower PM and PAH emissions have been achieved with Agrodiesel 15 than with Swedish Environmental Class 1 diesel fuel. A long-term test and a field test of one year with buses showed no problems. (Petterson 2005, Golubkov 2005).
Yeh et al. (2001) studied various esters, carbonates, alcohols, and ethers at 2 wt-% oxygen level, and found isodecanol as the efficient oxygenate in reducing PM emission. Also a paraffinic component (IsoparTM) reduced PM emission to some extent, but not as effectively as isodecanol. Janssen et al. (2009) reported that in advanced combustion systems 1-decanol could reduce soot emissions up to 90% depending on load point. All oxygen containing compounds do not effectively reduce PM emissions, for example, aromatic 1-phenyl ethanol and 1-cyclohexyl ethanol (Karas 1994).
Ethers, acetals, ketones
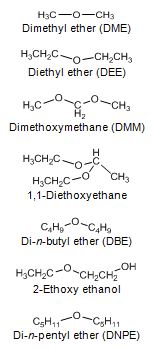
Diethyl ether (DEE) and di-methoxymethane (DMM) have been studied as diesel fuel extenders. Boiling points of these ethers are very low, 35 °C for and 42 °C. Acetal (1,1-dietoxyethane, derived from ethanol) is a light compound with a boiling point of only 103 °C and a flash point of -20 °C. Di-n-butyl ether (DBE) has a boiling point of 141 °C, which is almost within the distillation range of diesel fuel, whereas flash point is only 25 °C. DBE at 5 vol-% concentration resulted in a 5% reduction in PM emission with direct-injection car. 2-ethoxy ethanol has been found as an effective diesel fuel extender at 2.5, 5 and 7.5 wt-% blends in terms of exhaust emissions. Boiling point of 2-ethoxy ethanol is 135 °C. (Arteconi et al. 2011, Beeckmann et al. 2010, Subramanian et al. 2009, Nord 2005, Cheng et al. 2002, Delfort et al. 2002, Bailey et al. 1997).
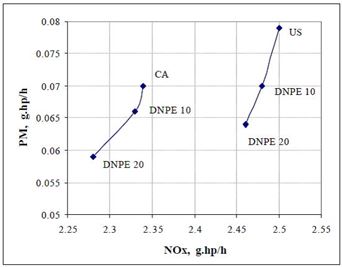
Mono-ethers give a good compromise between cetane characteristics and the behavior at low temperature. Di-n-pentyl ether (DNPE) has been reported to be a potential oxygenate for blending with diesel fuel. Cetane number of DNPE is high, 103 - 153, and other properties are diesel-like. DNPE is fully soluble in diesel fuel, whereas solubility in water is low. DNPE is 15 times more biodegradable than MTBE. DNPE could be produced, for example, from 1-pentanol, n-butane, or from methane via propylene oxide pathway. Favorable engine performance for DNPE has been reported. For the heavy-duty engine, PM, CO and HC emissions reduced by 15 - 20%, and NOx emission by 2 - 2.5% with fuel containinng 10-20% of DNPE (Figure 4). In the car tests, DNPE reduced PM emissions by nearly 10% without compromising other emissions, also under cold conditions. DNPE resembles high cetane paraffinic hydrocarbons as concerns engine performance and exhaust emissions. (Marchionna et al. 2000, Tejero et al. 2006, Murphy 1999, 2002, Martin et al. 1997, Giavazzi 1991, Pecci et al. 1991).
Figure 4. Trade-off between PM and NOx emissions by using DNPE as diesel fuel component for the heavy-duty engine. (Marchionna 2000)
Ryu et al. (2000) finds poly-ethers with high molecular weight as better diesel fuel components than mono-ethers. Dibutoxymethane (butylal) is soluble with diesel fuel. Its boiling point is 180 °C and flash point 62 °C. Cetane number of butylal is high (>74), but lubricity additive is needed. Diesel-butylal mixtures reduced engine exhaust opacity without increasing NOx emission when compared with diesel fuel. (Murphy 1999, 2002, Bertola et al. 2000).
Di-n-pentoxy methane (DNPM) has been found as favorable diesel fuel component reducing PM emission at a 25% blend in the tests with a passenger car. Boiling point of DNPM is 218 °C and cetane number is 97. (Giavazzi 1991).
Ten acetals and polyacetals at 1 - 2 wt-% oxygen contents were studied with a direct-injection car. The most significant PM reductions, 8-15%, were achieved with blends containing 5 vol-% of di-ethoxy-1,1-pentane, di-ethoxy-1,1-butane, di-ethoxy-1,1-propane or di(ethoxy-2-ethyl)polyoxomethylene. Diethoxy butane, which could be produced from ethanol and butadiene, has a high cetane number of 97, but a flash point of only 45 °C. (Delfort et al. 2002, McCormick et al. 2002)
Many other ethers have also been studied as diesel oxygenates. For example, 2-ethoxy ethyl ether (diethyl carbitol), trioxane (cyclic ether), and trimethoxy methane (McCormick et al. 2002, Murphy 1999, Yeh et al. 2001). Snamprogetti’s “oxy-diesel”, a poly-oxy-methylene, is achieved via oligomerization of dimethoxy methane (DMM) It is claimed to resemble diesel fuel and to be more biodegradable than MTBE. (Hart Diesel Fuel News 2001). MAN has tested oxymethylene ethers (OME) as diesel fuel additives. These are relatively easy to synthesize from methanol. (Lumpp et al. 2011). Boot et al. (2009) found good combustion behaviour for cyclohexanone.
Glycol ethers
Glycol ethers are produced from ethylene oxide (E-series) or from propylene oxide (P-series). (Glycol ethers online 2011).
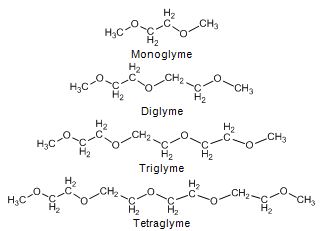
For diglyme, good engine performance, low smoke and low NOx emissions are reported. 14.4 vol-% diglyme blend resulted in a 20 - 40% reduction in PM emission with a light-duty truck. In addition, HC and CO emission reduced without increase in NOx, benzene, butadiene, formaldehyde and PAH emissions (Ren et al. 2007, Loganathan et al. 2007, Zhu et al. 2003). A mixture of 20/80 monoglyme and diglyme (CETANERTM, cetane number >100) at 15% concentration reduced PM by 42% and NOx by 8.8% with a direct-injection diesel engine (Hess et al. 2000). Glyme, diglyme and triglyme at 5 vol-% blends (around 2 wt-% oxygen) reduced PM, CO, and HC emissions, whereas NOx emission increased by 2 - 8% with a Euro 4 passenger car. Diglyme was the best oxygenate offering 13% PM reduction, while NOx increased only 2.5%. In another study, triglyme and tetraglyme reduced PM emission by 5 - 6.5%, and tetraglyme decreased NOx emission by 2.5%. 1 wt-% oxygen obtained with glymes resulted in 6 - 7% reduction in PM emission. (Kozak et al. 2007, Natarajan et al. 2001).
Nabi and Hustad (2010) studied diethylene diglyme and jatropha biodiesel at oxygen level of 2.3 wt-% with a Euro II engine. A PM reduction was close to 30% with both fuels. CO and HC emissions were reduced as well, but NOx increased slightly or remained unchanged.
Liotta et al. (1993) found glycol ethers more effective than alcohols in reducing PM emission at the same fuel oxygen level, however, at cost of increased NOx emissions. In the opposite, Yeh et al. (2001) found lower PM emission for isodecanol than for esters, carbonates, alcohols, and ethers at 2 wt-% oxygen level.
Tripropylene glycol monoethyl ether (TPGME), dibutyl maleate (DBM) and carbonates
Natarajan et al. (2001) reviewed 71 oxygenates, of which 8 oxygenates were selected for engine tests. The criteria was to achieve 7 wt-% oxygen content in blend by using in maximum 20 vol-% of oxygenate. Therefore oxygenates with oxygen content below 35 wt-% were excluded. From the selected eight oxygenates, tripropylene glycol monomethyl ether (TPGME) and di-butyl maleate (DBM) were deemed to be the most promising ones. The other selected oxygenates were methoxy-2-propanol, dipropylene glycol monomethyl ether, 2-ethoxy ethyl acetate, 2-ethoxy ethyl ether, di-ethyl adipate and tributyrin. TPGME and DBM are miscible in aromatic diesel fuel, but the former only up to 30 vol-% and the latter only up 10 vol-% in paraffinic diesel fuel. Addition of water may lead to phase separation. (Murphy 2002, Gonzáles et al. 2001).
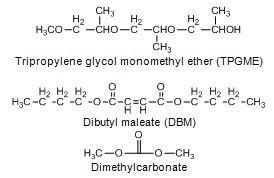
DBM and diethyl maleate reduced in-cylinder soot by 12%, and NOx by 5 - 8.5% in the tests with a single-cylinder engine, but not with a direct-injection light-duty engine (Natarajan et al. 2001). Stoner and Litzinger (1999) found maleates more effective than glycol ethers in soot and NOx reduction. This was thought to be due to delayed start of combustion. In the opposite, Mueller et al. (2003) found TPGME more effective than DBM in soot reduction with a single-cylinder engine. DBM produced ethylene, a soot precursor, in combustion.
With a passenger car, the best PM/NOx trade-off results were achieved diethyl maleate, DMC and DEC from a set of 7 glycols, 2 maleates and 2 carbonates at 5 vol-% concentrations. DEC was the most effective oxygenate in reducing PM emission, whereas CO and HC emissions increased. (Kozak et al. 2008, 2009). Delfort et al. (2002) found carbonates more efficient in reducing PM emissions than ethers and acetals (tetra-hydrofurfuryl derivatives) with a direct-injection diesel car. DEC, dipentyl carbonate and two ether carbonates resulted in 12-17% reduction in PM emission at 5 vol-% blends, and almost 30% reduction at 10 vol-% blends. Also other tests have shown reduced PM emissions for DMC blends, whereas contradictory NOx results have been achieved. (Keyu et al. 2002, Huang et al. 2003). Subramanian et al. (2009) found 2-ethoxy ethanol more efficient than DEC or diethyl ether at 2.5, 5, and 7.5 wt-% concentrations in terms of exhaust emissions.
Guo et al. (2005) synthesized methyl 2-ethoxyethyl carbonate (MEEC) by introducing an ether group to dimethyl carbonate. Smoke, NOx and CO emissions reduced substantially when 15-25 vol-% MEEC was added into diesel fuel in the tests with a single-cylinder engine. Viscosity of fuel decreased with addition of MEEC.