Emissions
For diesel engines, NOx and PM emissions are bottlenecks. These can be reduced by using emission control technologies or by using tailored fuels, such as oxygenates. PM reductions of 3 - 15%, and even 67%, have been observed at 1% oxygen level in diesel fuel. Soot-free combustion may be achieved at sufficient oxygen to carbon ratio, for example at 30 - 40 wt-% oxygen content. NOx emissions tend to increase slightly with oxygenates, depending on molecule and on engine characteristics. Carbonyl emissions may increase with oxygenates, but not necessarily if high cetane number is combined with over-lean mixture zone and high combustion temperature. In the automotive engines, the dominating process for NOx formation is oxidation of nitrogen in air at high temperatures (Zeldovich mechanism). Particles are formed in the rich zones in the fuel jet via pyrolysis of the fuel. (Boot et al. 2009, Gonzales 2001, Serdari 2000, Hess 2000, Chang 1997, Bertoli et al. 1997, Karas 1994, Hashimoto et al. 1991).
A number of mechanisms have been presented to explain reduction of PM emission with oxygenates. Oxygenated fuels bring oxygen in the combustion process. Many studies emphasize the differences between the functional groups of oxygenates. The role of oxygen entrained from ambient has also an impact, as well as the engine characteritics, load mode and test conditions. In older engines with fully mechanical injection systems, increase in fuel density and viscosity advanced injection timing leading to higher NOx emissions. In new electronically controlled injection systems fuel properties do not influence the start of injection. The load-dependent ability of oxygenates to reduce PM emissions might be related to changes in air-to-fuel ratio and combustion temperature. Flame temperatures of aromatic hydrocarbons are higher than those of paraffins and alcohols. Evaporation of alcohols cools the air-fuel mixture. Temperatures may be locally reduced due to higher evaporation heat of in the reaction zone where soot appears. Oxidation molecules may be available earlier in rich and high temperature zones enabling soot oxidation already in the phases of soot formation. High cetane number of fuel generally leads to decreased PM emissions. In some cases cetane number has been determining factor at lower loads, whereas oxygen content has been dominating at higher loads. The high cetane number and oxygenates could be beneficial at high EGR ratios. For some engines, oxygenates with low cetane number have led to low PM emissions. (Nabi and Hustad 2010, Janssen et al. 2009, Boot et al. 2007, 2009, Delfort et al. 2002, Bertoli et al. 1997, Giavazzi et al. 1991).
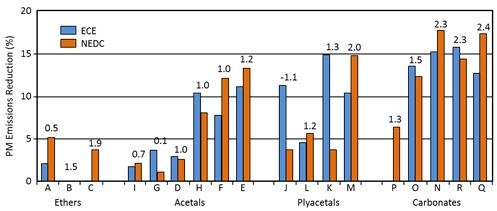
Delfort et al. (2002) concluded that the PM emission depends primarily on the functional group of oxygenate. Within each functional group, oxygen content is a key parameter based on a study with light-duty direct-injection car (Figure 1). The best performance was observed for fuels having the highest oxygen contents, but also some low-oxygen fuels reduced PM emission efficiently. (Boot et al. 2007, Bowman 2006, Delfort et al. 2002, Karas 1994).
Figure 1. The effect of oxygen is important within each chemical group of oxygenates (Delfort et al. 2002)
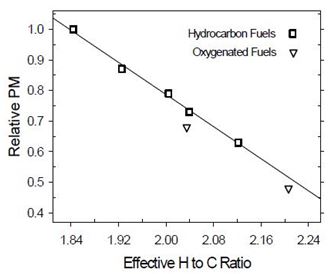
PM emission depends on hydrogen to carbon ratio for hydrocarbons fuels, whereas for oxygenated fuels PM emission is lower than could be explained by only carbon to hydrogen ratios of fuels (Figure 2). Strong carbon-oxygen bonds are assumed to prevent carbon from efficient soot formation. In principle, 30% of oxygen in esters is unavailable for eliminating soot precursors (one C atom per two O atoms), whereas C-O bonds protect one C atom per O atom. However, contradictory results have been obtained for these molecules. This could be explained if role of the C-O-C bonds was higher at premixed combustion, whereas role of the O-C=O bonds would be dominating at mixing-controlled (diffusion) combustion. Other structural parameters of molecules may also have an impact. Soot reduction efficiency is claimed to be highest when oxygen is in the middle of molecule, carbon chain is short (lower boiling point), no branches exist and when ethylene and methyl radicals are produced instead of larger alkenes. For high-molular weight esters, PM emission seems not to be dependent on branching. With oxygenated fuels, less soot may be achieved without significant differences in combustion phasing or heat release. A combination of oxygenated diesel fuel and emission control technologies may result in very low exhaust emissions. In general, non-aromatic fuels with simple chemical structure and short carbon-carbon chain burn cleanly. (McEnally and Pfefferle 2011, Boot et al. 2009, Xu et al. 2006, Mueller et al. 2003, McCormic 2002, Delfort et al. 2002, Natarjan 2001, Gonzales 2001, Yeh et al. 2001, Bertoli et al. 1997).
Figure 2. Relationship between hydrogen to carbon ratio of fuel and PM emission (Natarajan 2001)