Fuel properties
ISO has progressed standardization of DME fuel through TC28/SC4/WG13 since 2007. The DME standardization covers three categories: 1) home and industrial use, 2) blending with LPG and 3) fuel for diesel engines. A draft DME fuel specification ISO 16861:2015 is available for general DME use (Oguma 2018, IEA-AMF Task 47). The specifications for use of DME in vehicles are considered in the IEA-AMF Task 47. Properties and proposals for DME standards are presented in Table 1. Complete final requirements and standards are available from respective organizations. OBATE-M concept is not included in the Table.
Table 1. Selected properties and proposals for standards for DME (dimethyl ether, methoxymethane).
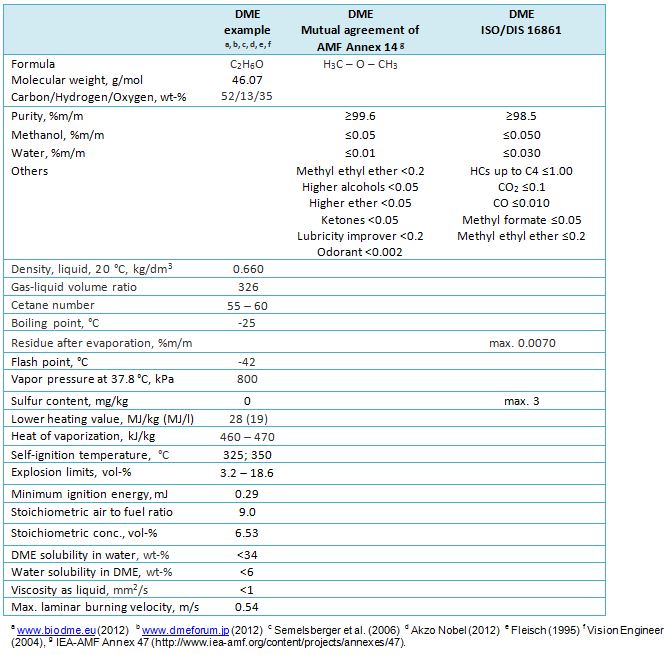
Lubricity improver additive and lubricity testing methods are discussion items in standardization of DME fuel for internal combustion engines (see “Engine” Chapter below). The ISO standard for DME use “does not deal with the possible additives necessary for specific end-use applications, for example, odorant typically added to heating fuel and lubricity improvers for DME used as replacement of diesel. Such additives are typically specified for the different end-use applications”. According to Oguma (2018, IEA-AMF Task 47), the test methods of lubricity will be “informative” in the DME fuel standards due to challenges in standardization of the special HFRR test methods for DME.
High cetane number of DME (55-60) makes DME a tempting fuel for diesel process. DME has been used as an ignition improver additive for methanol fuelled compression-ignition engines. DME is the lightest ether with a boiling point of only -25°C. It is a gaseous fuel at normal temperature and pressure, however, a mild pressure of approximately 6 bar is sufficient to keep it in liquid form. In this respect, DME resembles liquefied petroleum gas (LPG), which consists of propane and butane. Low viscosity and poor lubricity of DME is challenging for engine use. (Flesch et al. 1995, Karpuk et al. 1991). Vapour pressure of DME increases with increasing temperature, whereas liquid density decreases, respectively (Figure 1).
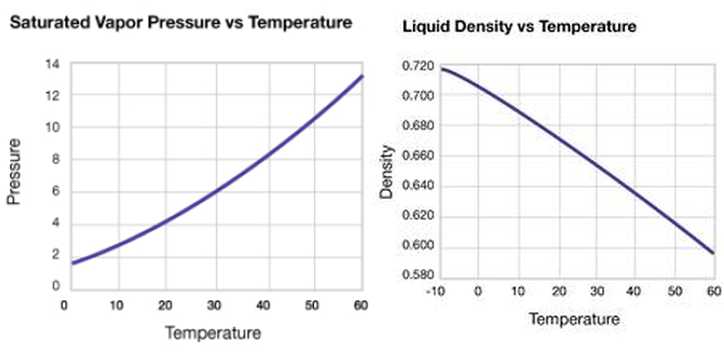
Figure 1. Vapour pressure and liquid density of DME changes with increasing temperature (Akzo Nobel 2012)
DME is polar, and consequently, miscible with water. Solubility of DME in water is up to 34 wt-%, and solubility of water in DME up to 6 wt-%. Alcohol can be used to avoid phase separation, for example, 6 wt-% of ethanol is sufficient to make all blends of DME and water to stay in a single, clear phase. DME is an excellent solvent. (Akzo Nobel 2012).


