Fuel properties
General information on fuel ethers at Oxygen+ website: https://globalfuelethers.com.
Molecular structures of the most common ethers MTBE, ETBE, TAME, and TAEE are as follows.
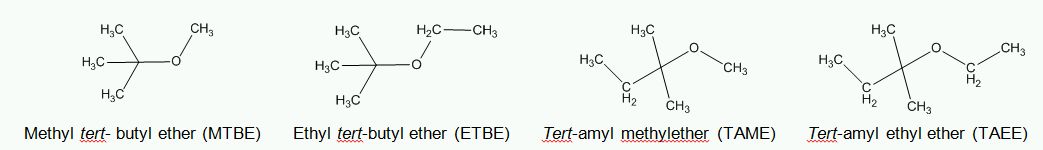
Figure 1. Molecular structures of MTBE, ETBE, TAME and TAEE.
Limitations for ethers are defined regionally in the legislation and standards for gasoline. In the U.S., gasoline-oxygenate blends are considered “substantially similar” if they contain hydrocarbons, aliphatic ethers or aliphatic alcohols other than methanol (up to 0.3 vol-% methanol or up to 2.75 vol-% with an equal volume of butanol or higher molecular weight alcohol). The fuel containing aliphatic ethers and/or alcohols (excluding methanol) must contain no more than 2.7 mass-% oxygen. This oxygen limit would represent about 17 vol-% ETBE content in gasoline. In Europe, the Fuel Quality Directive 2009/30/EC allows maximum 22 vol-% C5+ ethers in gasoline. Auto manufacturer's recommendations for fuel gasoline qualities in the WWFC 2006 edition states that ““Where oxygenates are used, ethers are preferred".
Typical properties of different ethers are shown in Table 1.
Table 1. Selected properties of different ethers considered as gasoline components.
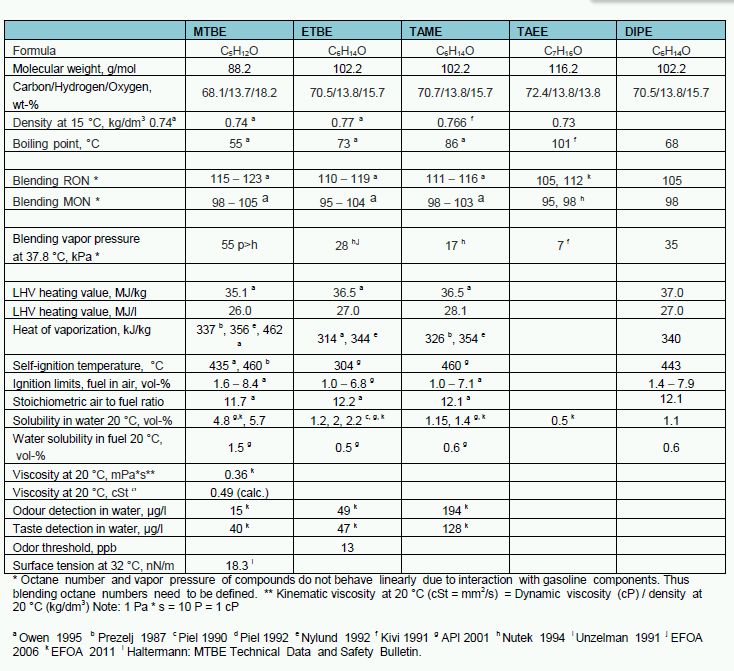
Gasoline consists of hundreds of different hydrocarbon molecules, whereas ethers are monomolecular compounds with narrow boiling points. Ethers are also aromatic-, olefin-, and sulfur-free compounds. Thus, they may improve gasoline composition by dilution effect. Refinery olefins are consumed in processing of ethers.
Sufficient knocking resistance, octane rating, is essential for proper operation of spark-ignition engine. Octane numbers of ethers are high, and thus they have been used as octane boosters in gasoline (Read more of octane numbers). For MTBE, ETBE, TAME, and TAEE blending research octane number (RON) is 105-123 and motor octane number (MON) 95-105. Alcohols tend to increase more RON than MON, while both octane numbers are excellent for fuel ethers (Figure 2).
Vapor pressures of ethers are low (Figure 2.) and ethers do not have azeotropic behaviour with gasoline and thus blending is predictable as regards volatility properties. This is a benefit for ethers when compared to methanol or ethanol (Wallace 2009).
Ethers affect mid-range distillation by increasing volume of distilled at 100 °C. On the other hand, heat of vaporization is at the same level for ethers as for gasoline, which may diminish the effect of ethers on driveability and cold-start emissions. According to Wallace (2009), addition of ETBE in the mid-range distillation could improve vehicle warm-up during cold engine operation without any drawbacks in hot driveability performance.
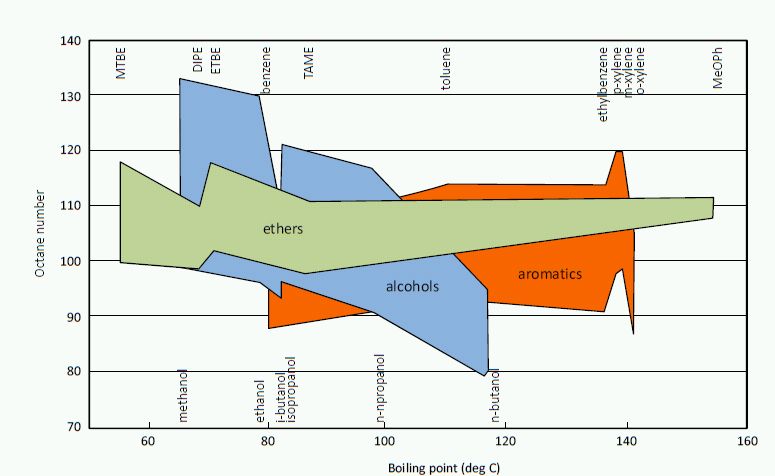
Figure 2. Octane numbers as a function of boiling point for oxygenates and aromatics (Oil Gas 1991).


